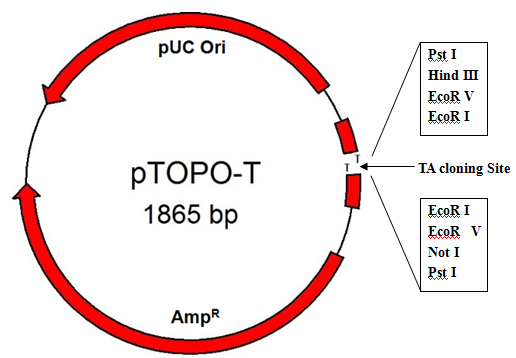
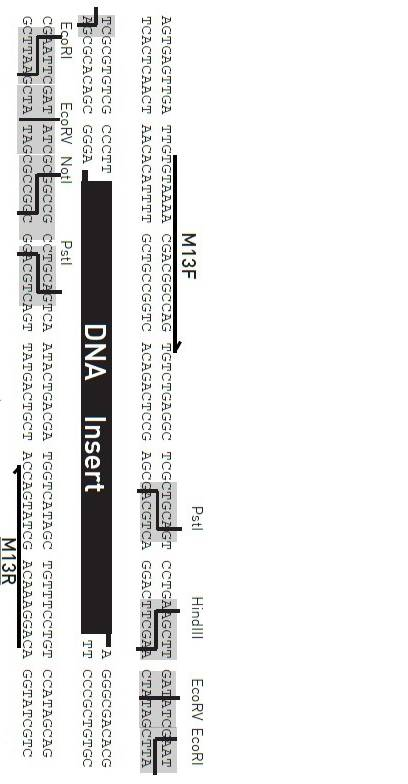
Principle
TA cloning kit can connect DNA fragments in an instant (a few seconds to a few minutes) and efficiently (close to 100%) under the action of Topoisomerase, so that PCR fragments are efficiently linked to carriers. TA cloning kit can connect fragments up to 10kb (that is, the insertion efficiency of 5kb fragments reaches more than 80%), which is a simple, fast, zero-background screening TOPO TA cloning vector.
PCR®2.1 Preparation
1. Prepare the solutions
|
Fresh PCR Solution/1uL 1000bp control |
0.5-8 uL |
|
pTOPO-T Vector |
1 uL |
|
10’Enhancer |
1 uL |
|
Sterile Water |
X uL |
|
|
10uL |
2. Ligation Reaction
After adding the reagent, gently blow the mixture with a pipette or flick the bottom of the tube to mix it.
Incubate at room temperature for 15 minutes. Longer incubation times increase the cloning efficiency.
3. Centrifuge
Collects all liquids at the bottom of the centrifuge tube based on low speed instantaneous centrifugation.